Paper Published! Raquel's work in Leonari group has been published on Chem
2023-11-14
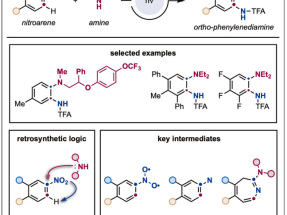
A strategy for ortho-phenylenediamine synthesis via dearomative-rearomative coupling of nitrobenzenes and amines
Ortho-phenylenediamines are aromatic molecules featuring two vicinal N-substituents with strong structural relevance to the development of bioactive materials. These derivatives are currently prepared from ortho-halogenated nitrobenzenes via multistep synthetic sequences. Here, we report a conceptually different approach where nitrobenzenes and amines can be directly converted into ortho-phenylenediamines without the need for ortho-halogenation and following stepwise synthetic manipulation. This strategy occurs under simple blue light irradiation and introduces an alternative retrosynthetic tactic whereby the amine coupling partner ‘‘seems’’ to displace the nitro group that shifts to its ortho position while being reduced and amidated in a one-pot process. Mechanistically, this process capitalizes on the conversion of nitrobenzenes into the corresponding single nitrenes, which undergo a series of N-insertion, electrocyclic ring expansion, amine addition, and electrocyclic ring contraction en route to the ortho-phenylenediamines.





