Research Project 9
State of the art
- Generation of (alkyl) radicals by photoredox catalysis is widely used but the adoption of uncharged easily available radical precursors is lacking
- Charged radical precursors (actually used as salts) have the disadvantage
to reduce the number of solvents possibly used due to solubility concern - Finding uncharged radical precursors that are easily oxidized by a
photocatalyst is challenging
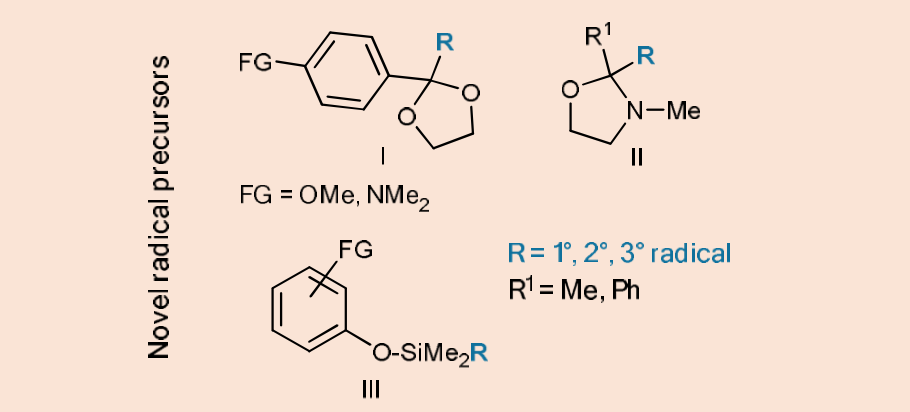 Scheme 9. New, uncharged radical precursors.
Scheme 9. New, uncharged radical precursors.
Progress beyond the state of the art
- Photocatalyzed oxidation of 2-phenyl-2-alkyl dioxolanes (I) may lead to the liberation of radicals in analogy with the 2,2-dialkyl analogues having, however, a prohibitive oxidation potential (Eox)
- 2,2-disubstituted N-alkyl oxazolidines (II) are conceptually new radical precursors having a very low Eox (ca. + 1 V vs SCE) that can be used for the generation of radicals, adopting a wide range of photocatalysts.
- The electron-rich aromatic ring in phenols protected with a silyl group (III) will assure an easily accessible oxidation Eox (ca. + 1.4-1,8 V vs SCE) and a facile release of the desired radical.
- The radical precursors will be easily prepared starting from the corresponding ketones (e.g. for I and II) or phenols (for III)
Key Objectives & Expected Results
Performance Indicators
- Photoredox catalyzed alkylations of electron-poor olefins, aromatics, etc. for the preparation of valuable compounds will be carried out both under batch and flow conditions in cooperation with the other partners.
- Innovative photocatalysts developed by the partners will be tested for radical generation.





