Research Project 7
State of the art
- 59% of small-molecule medicines contain at least one nitrogenated heterocycle, with piperidine being the most prevalent one (Scheme 7).
- Current approaches for the manufacture of these high-value materials require multi-step synthesis based on the introduction of functionality handles to allow the introduction of the N-heterocycle.
- The possibility to streamline the preparation of these materials to a single chemical reaction without the need of pre-functionalization is an unaddressed challenge in the area.
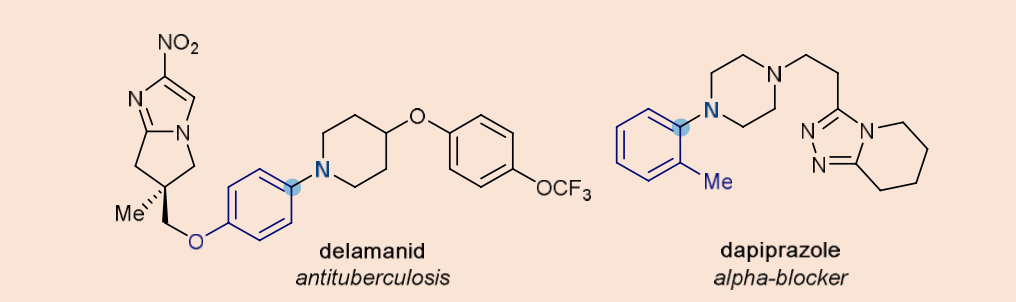 Scheme 7. Examples of nitrogen-containing medicines
Scheme 7. Examples of nitrogen-containing medicines
Progress beyond the state of the art
- We have recently developed a photocatalytic process for the installation of amines onto electron rich aromatics. This methodology requires the use of commodity chemicals like N-chlorosuccinimide and a mineral acid under visible-light irradiation.
- The main synthetic advantage is that it does not require the pre- functionalization of the aromatic coupling partner and therefore enables the installation of the amine moiety in place of a C–H bond.
- So far, this chemistry has been only applied to the amination of electron rich aromatics with alkylamines.
Key Objectives & Expected Results
Performance Indicators
- Identification of conditions for the use of amides, carbamates and ureas as N-radical building blocks. This will enable access to different nitrogenated motifs commonly used in bio-organic chemistry.
- Development of reaction conditions to enable reactivity at specific positions of aromatic coupling partners: ortho vs meta vs para.
- Demonstrate scalability of photocatalytic processes using batch-to-flow technology.





