Research Project 6
State of the art
- Late-stage modification of peptides and proteins represents a highly valuable tool to access functionalized non-proteinogenic analogues with tailored properties and/or improved therapeutic capabilities compared to their natural counterparts
- While transition-metal-promoted C-H functionalizations have emerged as a powerful tool in this area, the highly unreactive nature of Csp3-H bonds typically translates into harsh reaction conditions that are incompatible with multiple functional groups present in advanced targets and lack chemo-, regio- and stereoselectivity.
- In contrast, 1,n-hydrogen transfer (HAT) represents a potential useful entry to functionalize, in a site-selective manner, aliphatic C-H bonds via H-atom abstraction thus circumventing the pre-functionalization of substrates or the use of directing groups.
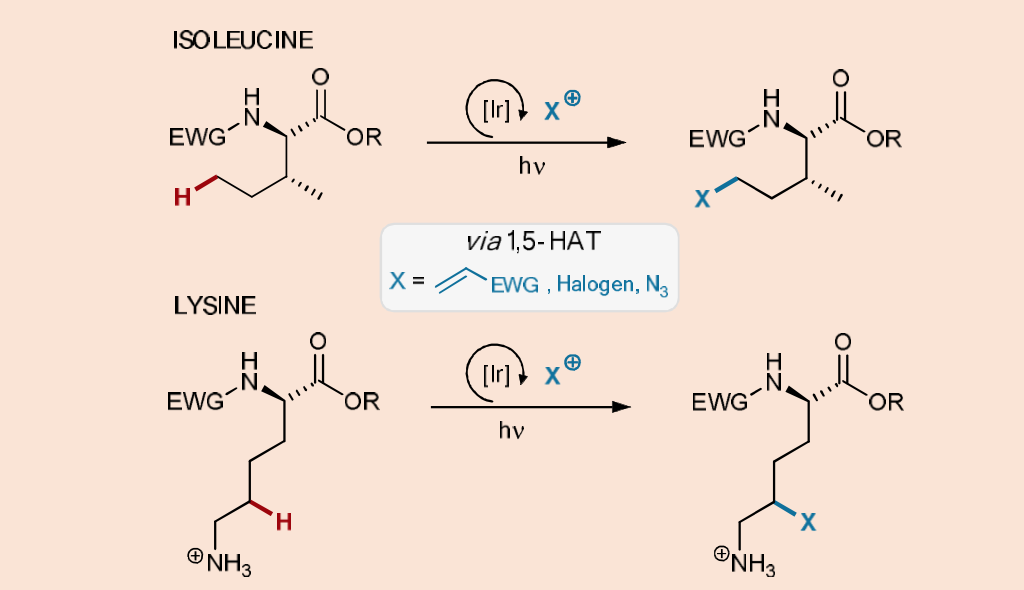 Scheme 6. Photocatalytic functionalization of isoleucine or lysine residues
Scheme 6. Photocatalytic functionalization of isoleucine or lysine residues
Progress beyond the state of the art
- Recently, our group has reported the successful generation of amidyl radicals under mild photoredox conditions that enabled remote functionalization reactions with saturated alkanes via 1,n-HAT processes.
- Since the use of fluorinated alcohol solvents can, via polarity reversal, deactivate the C-H bonds that are proximal to polar groups (such as NH or OH), we will tackle the functionalization of peptide substrates at C-H bonds that are remote from the backbone (and side-chain) polar groups.
Key Objectives & Expected Results
Performance Indicators
- Site selective functionalization of inert Csp3-H bonds with concomitant formation of new C-C and C-X bonds in a chemo- and regioselective fashion under water-compatible conditions.
- Demonstration that photochemistry can be applied to more complex and elaborated peptide substrates.
- Use React-IR and NMR in combination with advance kinetic processing software (Dynochem) to determine the mechanism of the reactions and the factors governing the selectivity.
- Construct predictability models of the reactions ́ selectivity using computational tools (DFT calculations) and kinetic data.





